 There are a number of ways to measure and analyze salt in food. Here's a breakdown of why we need salt in food, the affect it has on our health, and the different methods used to measure it. Scroll down to read the entire article, or click on a point below to jump to that topic.
There are a number of ways to measure and analyze salt in food. Here's a breakdown of why we need salt in food, the affect it has on our health, and the different methods used to measure it. Scroll down to read the entire article, or click on a point below to jump to that topic.
About Salt in Food
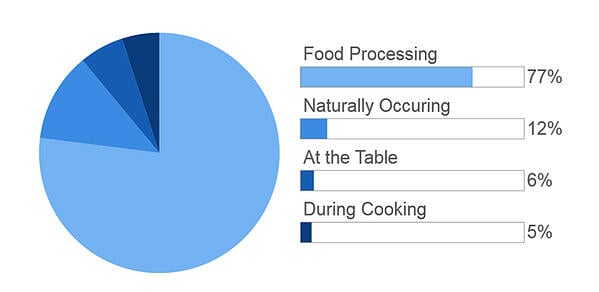
Sodium occurs both naturally and as an additive in food products. It is commonly added to food products in the form of sodium chloride (NaCl) but can also be added in other forms including sodium nitrite, sodium bicarbonate (baking soda), sodium benzoate, and monosodium glutamate (MSG). These compounds are added to foods to enhance flavor, act as a binder, and/or to inhibit microbial growth to extend the shelf life of a product.
It is estimated that up to 75% of our dietary intake of sodium comes from packaged or restaurant food.
The other main contributors of sodium are table salt, condiments, and other seasonings such as seasoned salt. It is important to note that not all salts contain sodium chloride. There are other salts, including potassium chloride (KCl) and calcium chloride (CaCl2). But generally speaking, sodium chloride is one of the most common salts added to food.
Sodium's Effect on Health
Sodium is a necessary mineral for all of us. It is necessary for the nervous and muscular systems to function. It is found mainly in the blood and extracellular fluids. The amount of sodium in the blood has an impact on blood pressure. For example, an increase in the sodium levels of the blood will increase blood volume. An increase in blood volume will increase blood pressure.
There are many detrimental effects of high blood pressure including an increased risk of cardiovascular disease, congestive heart failure, and kidney disease.
The major source of our sodium intake is from sodium chloride. The Food and Nutrition Board for the Institute of Medicine states that for individuals age 9-50, the adequate intake (AI) level for sodium is 1.5 grams per day. This is not to be confused with recommended daily allowance. The amount of sodium required (AI) is a general statement.
There are many examples where a higher sodium intake is needed. For example, an athlete that sweats a lot will lose higher amounts of sodium as they perspire as compared to a person with a sedentary lifestyle.
The Food and Nutrition Board for the Institute of Medicine also states that the tolerable upper level (UL) intake for sodium to prevent changes in blood pressure for individuals 14 and older is 2.3 grams per day, which is equivalent to approximately 1 teaspoon of table salt (NaCl).
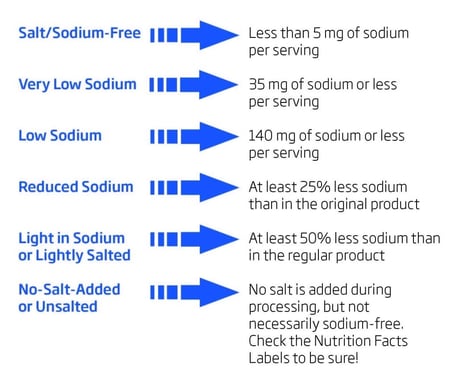
The amount of sodium in processed and manufactured foods must be measured and indicated in the Nutrition Facts portion of the label. Sodium is listed along with the associated percent daily value (%DV) of sodium in one serving of a food. A 100% DV is less than 2.4 grams/day. Foods can also be labeled according to the amount of sodium per serving for quick identification.
This table shows the guidelines that a company must follow for labeling.
Methods of Sodium Analysis
There are many different methods available for determining the sodium (salt) content of food, with each method having its own advantages and limitations. The selection of the method to use is an important decision to make when designing a quality assurance plan.
Considerations in determining the appropriate methods include equipment cost, accuracy desired, and experience level of the person performing the test. The most common measurement methods for determining sodium salt content include:
Method 1: Refractometry
This method determines the salt content of a substance based on the refractive index. The refractive index is determined by passing a light through a prism into a sample and measuring how the light bends. Refractometers determine the critical angle of a sample. The critical angle is the angle at which no light is refracted and all light is internally reflected.
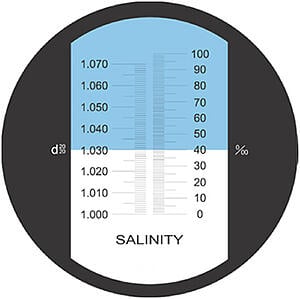
Refractometry can be used to determine a wide variety of parameters including sugar, propylene glycol, gelatin, and salt.
Each refractometer is based on the effect of density and temperature on the refractive index for a specific measured parameter. The refractive index is converted to a measurement unit such as % Brix (soluble solids as sucrose) or % salt.
There are two types of refractometers: digital and mechanical.
Mechanical Refractometers
With mechanical refractometers, the sample is placed on a prism, and the user looks through an eyepiece to observe the "shadow line" to determine this critical angle. Since temperature greatly affects refractive index, temperature compensation is achieved using bimetal strips that move the lens or scale as they expand or contract due to changes in temperature.
Manual refractometers are a low-cost investment but have limited accuracy due to the subjectivity of determining the "shadow line," variations in ambient light, and limited temperature compensation.
Digital Refractometers
Digital refractometers utilize an internal light source at a fixed wavelength. This internal light passes through a prism and into the sample and an internal light detector identifies the critical angle.
Digital refractometers remove the subjectivity of determining the shadow line manually and have improved temperature compensation due to the use of pre-programmed algorithms; they can also perform measurements over wider temperature ranges at a low-moderate price investment
Refractometers are attractive due to their low startup cost and that they use no chemical reagents. A small sample is all that is needed. This makes them ideal for quantitative use in simple solutions, such as a salt brine solution. However, this method is not specific to salt. Other substances present in the sample will alter the refractive index. These substances include fats, sugars, and minerals.
Method 2: Electric Conductivity (EC)
Table salt dissociates as two ions in solution: sodium and chloride. Since ions are charged particles, electricity is conducted more easily. As a result, an electrical conductivity (EC) meter can be used to estimate the amount of salt dissolved in solution. Once an EC measurement is obtained, a conversion factor specific to salt must be applied in order to get the amount of salt in a solution. Many meters have this calculation built-in.
There are two main types of probes used in measuring conductivity in foods: two electrode probes and four-ring probes.
Hanna Tip: Remember, EC is able to tell you the concentration of ions in solution, but not what those ions are. If you have a more complex sample, another form of measurement i.e. titration would be better suited to your application.
Two Electrode Probes
Two electrode probes are made from two electrodes spaced a known distance from each other. This distance is called the cell constant. A voltage is then applied to the two electrodes and current is measured between them. Ultimately the resistance can be determined, which can be used to calculate the conductivity.
Two electrode conductivity probes have the advantage of requiring a low sample size and are generally less expensive than other conductivity technologies. However, different ranges of salt require different cell constants using two electrode probe sensors. This means that multiple sensors are required for different ranges.
Four-Ring Probes
Four ring conductivity sensors use four ring electrodes. Two of these rings are sensing electrodes; the other two are drive electrodes. The drive electrodes apply an alternating voltage that induces a current in the solution. The sensing electrodes measure the voltage drop and the measurement is converted to electrical conductivity.
Four-ring conductivity sensors have wide measuring range and can cover virtually all food samples. However, these probes generally require more sample for the electrodes to be fully submerged due to the space required for all four-ring probes. These probes also tend to be more expensive, partially due to the platinum required for their manufacture.
Conductivity represents an attractive option for a QC setting for its ease of use and affordability. However, conductivity probes measure conductivity, not specific salts. This means that any ions (e.g. calcium, magnesium, etc.) will interfere with measurements. Thus, conductivity measurements only estimate the salt content rather than provide an exact value.
 Method 3: Ion-Selective Electrode (ISE)
Method 3: Ion-Selective Electrode (ISE)
The third method for determining salt content in food is with the use of an ion-selective electrode. This is often referred to as an ISE.
An ISE is a chemical sensor used to determine the concentration of a specific ion in a solution. In sodium ISEs, the tip is a sodium-sensitive glass bulb. Like a pH electrode, ISEs obey the Nernstian response, which allows us to correlate a millivolt (mV) reading to a concentration value.
Hanna Tip: ISEs require care to ensure accurate measurements.
The glass bulb of the sodium ISE must be hydrated at all times in an electrolyte solution. In addition, the electrode bulb needs periodic etching to ensure that a fresh layer of sensing glass is exposed prior to measurement.
The ISE must be calibrated daily in order to ensure accurate measurements. Calibration standards should bracket the expected concentration of the sodium content of the food. For example, one calibration standard should have a higher concentration than the expected value, and the other a lower concentration.
Ionic strength adjuster (ISA) must also be added in a fixed ratio to both calibration standards and samples for accurate readings. Electrode response is affected by ion concentration and ion activity. The ISA standardizes ion activity between calibration standards and samples, ensuring that changes in the electrode response are based on changes in ion concentration. Once calibration is complete, measurements of liquid or solid samples can be performed. Solid samples require pre-treatment in order to make a slurry that can then be measured with the probe.
Sodium ISEs are specific to sodium measurement and are prone to little interference. The startup cost to measure sodium with an ISE is moderate. A pH meter with an ISE concentration readout or a dedicated sodium ISE meter is needed along with the ISE, ISA, and calibration standards.
Method 4: Titration
Titration is the most common method of salt analysis by a food manufacturer with an in-house laboratory.
A titration method is referenced by organizations including AOAC for a variety of food products including cheeses, meats, and vegetables. A titration is a procedure where a solution of a known concentration (titrant) is used to determine the concentration of an unknown solution (analyte).
Results are calculated based on the amount of titrant used to reach an endpoint. An endpoint can correspond to a color change with the use of a chemical indicator, or detection with a potentiometric sensor, such as a chloride or silver ISE.
Manual Titration: Mohr Method
One way to determine salt content by titration is by the Mohr method. Historically, the Mohr method is a manual titration method using silver nitrate as a titrant and potassium chromate as a color indicator. In this titration, a volumetric burette is used to manually add silver nitrate titrant to a sample that has chloride analyte as the indicator.
The reaction between the silver and chloride ions produces an insoluble silver chloride (AgCl) precipitate. The silver nitrate is added until all of the chloride ions have reacted with the silver nitrate. At this point, any additional silver nitrate will be in excess resulting in the presence of silver ions.
 The silver ions will then bind to the potassium chromate color indicator to produce a red color in solution. This signals the endpoint of the titration. The chloride concentration is then calculated from the volume of silver nitrate that was added and then used to infer sodium or sodium chloride content.
The silver ions will then bind to the potassium chromate color indicator to produce a red color in solution. This signals the endpoint of the titration. The chloride concentration is then calculated from the volume of silver nitrate that was added and then used to infer sodium or sodium chloride content.
Hanna Tip: Manual titration relies on subjective interpretation of the color indicator change.
For this reason, this method is prone to overestimating the salt content. An additional challenge with a manual titration is the accurate measurement of the titrant that is used.
The investment to perform a manual titration with silver nitrate titrant, color indicator, volumetric burette, and other necessary glassware is very low.
Automated Titration: Potentiometric Method
Titration with silver nitrate can be automated with a potentiometric titration system. A titration system for salt analysis is equipped with an ISE or silver billet electrode. ISEs are sensitive to the concentration of either chloride or sliver ions. Silver billet electrodes provide a lower maintenance option that meets industry standards. Both electrodes are used to monitor the solution for a change in the mV potential as a result of pertinent ions being either consumed or in excess.

HI5148B Silver Billet Electrode
Potentiometric titration systems automatically control titrant dosing and endpoint detection. Automatic endpoint detection increases titration precision and accuracy by eliminating the human subjectivity that is associated with a titration using a visual indicator. Instead of monitoring for a color, the titrator determines the endpoint by measuring changes in mV potential. It is important to note that there is no calibration of an ISE or of a silver billet with potentiometric titration, since it is an abrupt mV change that is important.
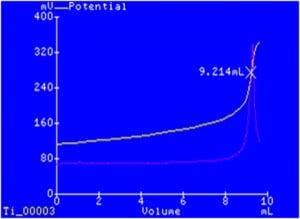 The automated dosing system of a potentiometric titrator also increases precision due to the ability to dispense and measure finite amounts of titrant.
The automated dosing system of a potentiometric titrator also increases precision due to the ability to dispense and measure finite amounts of titrant.
Many titration systems feature the ability to dynamically dose a titrant. Dynamic dosing allows the meter to control how much titrant is dosed based on the progress of the titration. Larger doses are dispensed in the beginning of the titration, with progressively smaller doses being dispensed as the endpoint is approached. This saves time and reduces the likelihood of overshooting the endpoint.
Automatic titrators will also perform all of the necessary calculations and display the results in the concentration units desired. The other benefits of an automatic titration system include the ability to generate reports for traceability and the option to perform other titrations including acidity.
Conclusions
Measurement method selection is one of the most important steps in establishing a protocol for monitoring salt in foods.
Here are the options in summary:
Refractometry
Refractometers are the easiest to use with low equipment cost and no chemicals required. They are not selective to sodium chloride and, therefore, can only be used for quantitative measurements in a solution that only changes in sodium chloride concentration.
Conductivity
Conductivity is an easy method to use and it is affordable. However, conductivity probes do not measure specific salts, so any ions (e.g. calcium, magnesium, etc.) will interfere with results. Conductivity is best for spot checking based on an estimated salt content rather than an exact value.
Ion Selective Electrodes
The sodium ISE is highly beneficial due to its high accuracy and because it directly measures sodium. The other methods discussed only infer sodium from another measurement. However, the required daily prep time for calibration and electrode care is high and requires excellent laboratory technique in order to obtain accurate measurements.
Titration
In both manual and potentiometric titrations, sodium content is inferred from chloride concentration. This can be problematic for samples that also contain other chloride salts and not just sodium chloride. For example, if magnesium chloride and/or calcium chloride were added in addition to sodium chloride, then a chloride titration will overestimate the sodium content.
Manual titrations lack accuracy and repeatability due to the subjectivity of determining the titration endpoint from a color change and the coarse dosing resolution of manual burettes.
Automatic titrators remove these limitations and provide for a highly accurate and precise measurement. However, the investment is highest as compared to the other mentioned methods.
The ideal method can change depending on the specific product but, in all cases, all available methods should be reviewed for their ease of use, accuracy, and costs. The measurement method selected prior to analysis is as crucial as the testing of the product itself.
Editors Note: This post was originally published in May 2016 and has been updated for accuracy and comprehensiveness.
As a leader in innovation Hanna Instruments developed the HALO Wireless pH Meter, which uses Bluetooth Smart Technology to connect to Apple and Android devices running the Hanna Lab App.
Continuing with this tradition, the Hanna Instruments Blog is devoted to sharing the latest in product overviews, how-to guides, and industry specific news to our ever-growing audience.
Contact us at sales@hannainst.com.



