
It’s easy to think about oxygen being in the air we breathe, but what about the liquids around us? We are not the only organisms that need oxygen to survive. Oxygen plays a major role in food preservation, water management, and environmental monitoring. It is important to remember that oxygen doesn’t just float around in the atmosphere but, it can also be present in liquids. This makes dissolved oxygen a key measurement parameter for many industries.
In this blog we will cover:
- What is dissolved oxygen?
- Why test for dissolved oxygen?
- What affects dissolved oxygen?
- How do we measure dissolved oxygen?
- Titration
- Galvanic
- Polarographic
- Optical Dissolved Oxygen
- Which instruments are best for measuring dissolved oxygen?
Why test for dissolved oxygen?
Why you would test for dissolved oxygen truly depends on the industry you are in. For instance, the reasons why a winemaker will test for DO in wine and the reasons a lab technician regularly monitors DO in a water sample are very different.
Dissolved oxygen is a vital parameter in the environmental monitoring of water quality. It is a great indicator of the general health of the ecosystem. As more organisms die, and eventually decay, it causes a bacterial growth spike. This spike results in an increase in DO use, and a decrease in the overall DO levels. Drops in DO can be enough to cause dead-zones to form, where all of the aquatic plants and animals in a body of water die off. Monitoring DO in aquaculture is done for similar reasons as well.
Laboratory measurements of DO can be direct, but it can also be used to monitor BOD, OUR, and SOUR. BOD (Biological Oxygen Demand) is used to help determine the biodegradable organic matter in water. This gives operators an idea of the general water quality, as well as the level of pollution in the sample. OUR (Oxygen Uptake Rate) is a quicker test than BOD that can be used as an indicator of biological activity of the sample. This is done by monitoring the oxygen consumption in the water. SOUR (Specific Oxygen Uptake Rate) is the amount of oxygen needed in mg/L microbes to consume one gram of food (waste). SOUR is usually monitored in sludge treatment.

What affects dissolved oxygen?
Temperature
Temperature is one of the biggest factors that directly affects dissolved oxygen. Temperature itself is a measurement of the energy of motion within a system. As temperature increases, particle motion increases. When temperature climbs, the particles, molecules, atoms, etc. get more and more energy. This energy makes them bounce around more rapidly. As these particles bounce around more, they collide with each other and can break the bonds that hold them together. DO particles can then have the bonds that hold them in the liquid break, and they can bounce out of the solution. Therefore, the higher the temperature, the lower the DO concentration. Inversely, as temperature decreases, particle motion decreases, and therefore DO concentrations go up.
Pressure
Pressure when talking about dissolved oxygen, refers to atmospheric pressure. Have you ever been at sea level and then traveled to a place of higher elevation such as Denver, CO? You may have noticed that you feel a bit lighter, but also that the air feels “thinner”. There is less atmospheric pressure pressing down at that altitude. It probably took a day or two for you to adjust to the oxygen and pressure differences. When atmospheric pressure decreases, the partial pressure of oxygen decreases too. As altitude increases, the concentration of DO decreases since there is not as much pressure keeping the oxygen diffused into the liquid. As atmospheric pressure increases (i.e. going back towards sea level), the partial pressure of oxygen increases as well. So, as altitude decreases, the concentration of DO increases.
Salinity
Salinity can also affect the amount of DO in a solution. This goes back to chemistry; how certain molecules can carry different types of charges. The charge that a salt molecule carries is very attracted to the water molecules, and is more likely to be dissolved into a solution. Oxygen isn’t as attracted to the water molecules in a solution if salt is present. This is due to the fact that salt will bump oxygen out of a solution since there just isn’t enough room. As salinity increases, DO will decrease.
Humidity
Humidity or water vapor can affect DO concentration, and the calibration of certain DO measurement technologies. When you have an increased humidity level, you have an increased partial pressure of oxygen, and therefore you have an increased level of dissolved oxygen.
How do we measure dissolved oxygen?
We can measure dissolved oxygen through iodometry, colorimetry, electroanalytical methods, and luminescence.
Iodometry
Iodometry, is a form of titration. Titration is where you use one substance that you know the concentration of (titrant) to determine the concentration of another substance (your sample). An iodometry titration is a titration method where the appearance or disappearance of iodine is used to signal the end of the titration. The titration used for determining the concentration of DO is called the Winkler Method. This provides a “one-time measurement” of your sample.
Colorimetry
Colorimetry is a measurement of color. We use chemical reagents to react with the DO sample, and they form a particular color. The intensity of the color is directly proportional to the amount of DO in your sample. The reagents used in colorimetric tests are similar to a modified version of the Winkler Method. This method, just like iodometry, provides a snapshot of what is going on in your sample with DO.
Electroanalytical Methods
Electroanalytical methods are quite simply, dissolved oxygen probes. The two types of probes that use this type of chemistry are called galvanic and polarographic, and they work based off of oxidation-reduction (redox) reactions. Either probe will provide continuous, live-time measurements for monitoring your samples. Before we discuss the electrodes, first let’s do a short review of the redox reactions.
Redox Reactions, also known as oxidation reduction reactions, occur when you have an exchange of electrons between two substances. Oxidation and reduction are two halves of a whole reaction; you cannot have one without the other. The oxidizer performs oxidation on the reducer, and the reducer performs reduction on the oxidizer.
- Galvanic probes are membrane probes, and behave like a battery. The probes have two parts that produce the battery behavior (producing a voltage). In the cap of the electrode there is a very thin membrane. This membrane is important as it is permeable to gasses and allows them through, but it keeps out everything else. As oxygen passes through the membrane, it dissolves into the buffered electrolyte inside the probe cap. The oxygen is then reacted at a part of the electrode called the cathode, and it is given an electron. The electron is supplied by another part of the electrode called the anode. This reaction and the exchange of electrons causes a voltage to be generated between the cathode and the anode of the probe. Once the current is formed, the meter the probe is attached to converts the reading into a DO concentration unit.
- Polarographic Probes work a bit differently than galvanic probes, while still being membrane probes. Instead of behaving spontaneously like a battery, there is a voltage applied between the cathode and the anode. This supplied voltage works as a catalyst to drive the oxygen reaction. Just like the galvanic probes, the electrode has a membrane that allows oxygen through into the unbuffered electrolyte. Once the oxygen hits the cathode, it gains an electron. This causes a current and the dissolved oxygen concentration is determined. Due to the applied voltage, these probes require a warm-up time prior to use.

Optical Dissolved Oxygen
Optical Dissolved Oxygen still uses a probe to measure the dissolved oxygen, but instead of monitoring a reaction, the probe and meter monitor luminescence. Simply, there is a blue light emitted by the probe, and the blue light excites the light sensitive material in the probe cap. As the material calms back down, it releases a red light, and it is measured when it hits a light sensor. If dissolved oxygen is present, it will suppress the red light. The intensity, lifespan of the light (decay), and frequency of the red light are all dependent on the concentration of oxygen. Optical dissolved oxygen probes are also able to provide a continuous measurement of your sample. Optical DO probes are a bit more affected by humidity than other probes.
Which instruments are best for measuring dissolved oxygen?
Now that you know a bit more about DO as well as why and how it is measured, you may be wondering which instruments are best for testing. You first need to decide whether you require a portable meter or a stationary benchtop meter.

Portable Meters
Portable meters provide the flexibility of being able to test in multiple locations, while still maintaining high levels of accuracy. These meters can vary greatly in design, testing method, and function. Some portable meters are designed as dedicated meters that only test one parameter, while others can test samples for more than one parameter at once. Take a look before purchasing, as some meters have the added benefit of being waterproof.
There are a variety of options that allow you to find the perfect fit for your testing needs. These portable meters can work with either/or the colorimetric method, a galvanic probe, a polarographic probe, or an optical DO probe. It truly depends on your preference, sample matrix, and the level of desired accuracy. Calibration of galvanic and polarographic probes are performed with either a single point and two-point calibration. This can be done in zero oxygen solution, or in 100% saturated air. Not to worry, there is a practical calibration beaker included to make the process even easier.
Hanna offers a variety of meters to meet your portable testing needs; from dedicated DO meters, such as our HI98193 Waterproof Portable Dissolved Oxygen and BOD Meter (perfect for demanding applications) or our newest addition the HI98198 Optical Dissolved Oxygen Meter (which makes DO testing more reliable and hassle-free), to our multiparameter meters such as the HI98196 Multiparameter pH/ORP/DO/Pressure/Temperature Meter (to meet all your water quality testing needs.
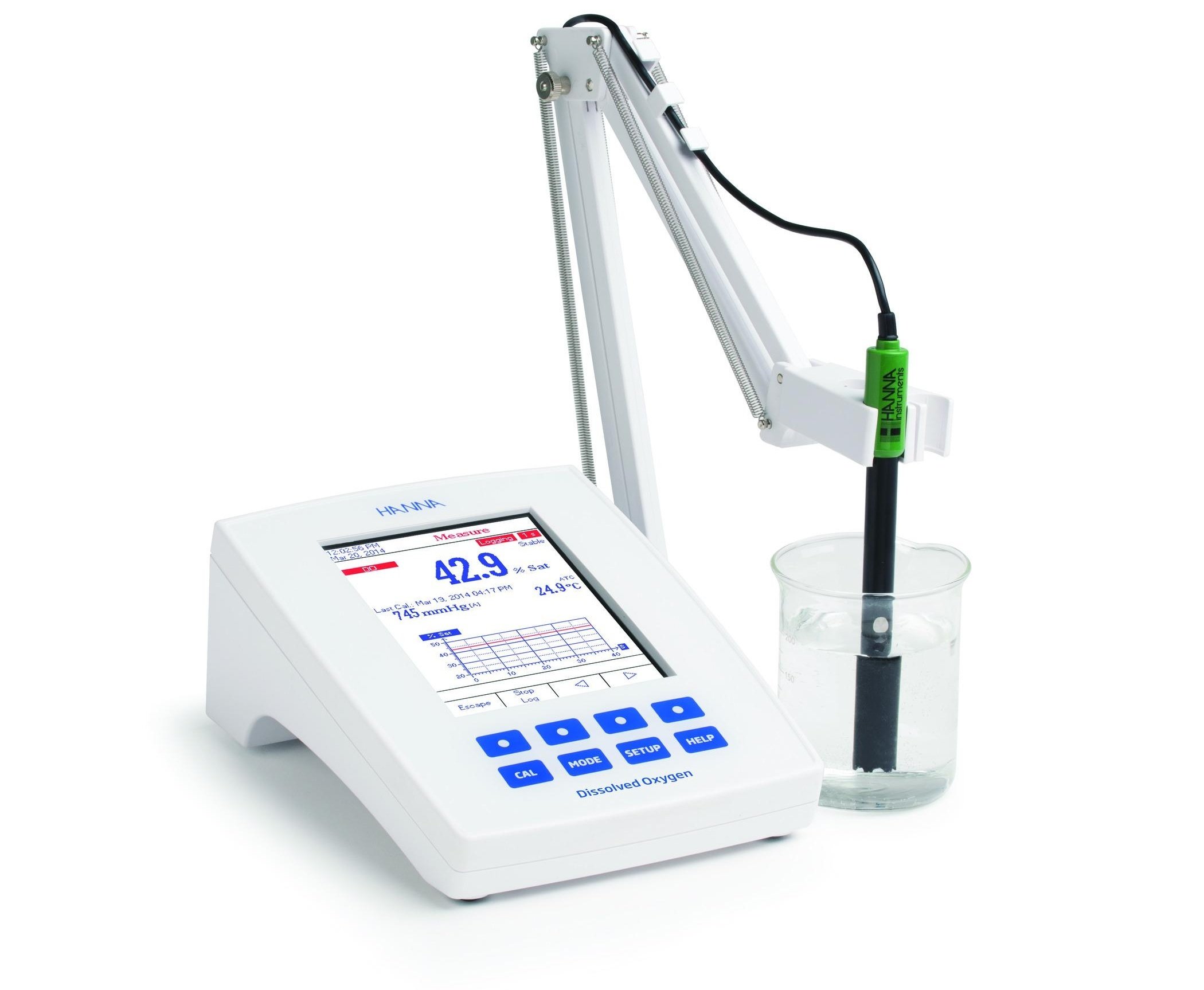
Benchtop Meters
Benchtop meters, just like portable meters, are varied and provide many options to meet what is required for the particular application. When looking into benchtop meters, it is a good idea to keep the amount of space you have in mind. Some meters such as a benchtop photometer can require more room than a meter that uses an electroanalytical probe. On the opposite end of the spectrum, there are also dissolved oxygen benchtop meters with zero footprint. Zero footprint meters can save you valuable benchtop space by being mounted on the wall.
Some benchtop meters are dedicated parameter meters. If only dissolved oxygen needs to be tested, this is an excellent economical option, such as our HI5421-01 Laboratory Research Grade Benchtop Dissolved Oxygen and BOD Meter. The integrated temperature and barometric sensors allow for temperature and barometric pressure compensation.
If there is the potential for testing other parameters down the road, it would be recommended to choose an expandable meter. This generally means that the initial meter is purchased as a package with the dissolved oxygen probe, and then other probes such as pH can be added down the road. Hanna offers the HI2040-01 edge® Multiparameter DO Meter. This meter and probe allow for DO in-situ testing in narrower environments, as the probe is slimmer.
Both portable and benchtop dissolved oxygen meters can compensate for the main parameters that affect DO readings (temperature, pressure, salinity, humidity). If you need to report your data, choose a meter that has the capability to log and to offload files onto a flash drive or computer.
While samples may be complex, measuring your dissolved oxygen doesn't have to be!
Use this guide to help you with the what, why, and how of dissolved oxygen testing to help get you started. For help in choosing the best option for your dissolved oxygen testing needs, talk to a Hanna specialist today.
That's why we've dedicated our blog as a helpful resource for you to use! Catch up on the latest products, explore industry trends, discover testing tips, learn how to improve results, and more. Got questions? Email sales@hannainst.com.

