 An automatic potentiometric titrator pays for itself within the first few weeks of use by removing subjectivity, increasing accuracy, reducing testing times, saving labor, decreasing chemical consumption and costs, and increasing the precision of bath analysis.
An automatic potentiometric titrator pays for itself within the first few weeks of use by removing subjectivity, increasing accuracy, reducing testing times, saving labor, decreasing chemical consumption and costs, and increasing the precision of bath analysis.
Remove Subjectivity by Using a Probe Instead of Your Eyes
Using a color indicator for manual titrations can be fast and simple, but there is a trade-off between time and accuracy. Color indicators are chemical dyes that change color based on the properties of a solution.
These color indicators are often used during manual titrations as an indicator of the consumption of a certain chemical, or the presence of a chemical in excess. The color change correlates to the titration endpoint.
What becomes tricky is where this color change occurs with respect to the eyes of the technician. Questions like “How pink is pink?” or “What shade of orange between yellow and red should I stop at?” are common.
This problem compounds when trying to detect this color change in a colored sample, such as a green nickel bath. These kinds of samples can be challenging even for skilled laboratory analysts to consistently reproduce, and are near impossible for someone who is colorblind.
How Potentiometry Works
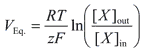
Figure 1: The Nernst equation allows us to correlate ion activity to a measurable voltage.
In a potentiometric measuring system, a titration endpoint is determined based on a change in potential in the solution. A meter and sensor accurately determine the millivolt (mV) potential of the sample solution. The sensor, such as a pH, ORP, or ion selective electrode, behaves according to the Nernst equation.
The type of sensor used will determine which ion(s) in the solution are measured. The inner reference potential of the electrode’s cell is compared to the outer membrane potential. During a titration, the activity of the ion being titrated changes as the titration progresses. The titration endpoint can be detected by determining the point where the maximum potential change occurs.
Using Ion Selective Electrodes to Monitor Nickel Concentration
 Nickel can be determined in plating baths by titration with EDTA, like our EDTA 0.02M, 1L - HI70449. As EDTA is added to thesample, the nickel becomes bound; the endpoint occurs when the nickel is completely bound by EDTA.
Nickel can be determined in plating baths by titration with EDTA, like our EDTA 0.02M, 1L - HI70449. As EDTA is added to thesample, the nickel becomes bound; the endpoint occurs when the nickel is completely bound by EDTA.
A nickel titration can be determined potentiometrically. However, nickel is a special case in that a sensor to directly detect ion activity is not commercially available. Instead, the nickel concentration can be determined in a titration by monitoring the displacement of copper by nickel with a cupric ion selective electrode (ISE).
First, the pH of the sample is buffered to approximately pH 10. Next, a small amount of copper EDTA (CuEDTA) is added to the sample. At pH 10, nickel preferentially binds to EDTA, displaces the copper, and results in free copper ions in solution.
We then titrate with EDTA. As the titration progresses, EDTA first binds the nickel ions in solution. Once all the nickel is bound, the EDTA then react with the free copper ions in solution. When this happens, the activity of the copper ion drastically decreases, which is detected by the cupric ISE. This signals the titrator to detect the endpoint.
With potentiometry, we are monitoring the actual activity of the ion we are trying to measure rather than looking at a color change with our eyes. Tracking the titration this way allows the reaction to be monitored in a consistent manner that eliminates subjectivity and increases accuracy and consistency between analysts.
Increase Precision by Using Automatic Dosing

You might ask, “Why is this important?” Well, getting repeatability between shifts and technicians ensures consistent baths and a quality product. The risk of human error is drastically decreased.
Titrations by hand are tedious and the endpoint can easily be overshot. A manual burette stopcock can only dispense one drop (~50 µL) at a time, and it takes skill to do so. This is not the case with automatic titration. An automatic titrator can dose down to 5 µL per dose with a standard 25 mL burette installed, ensuring that the endpoint is precisely detected every time.
Automation also helps increase accuracy and repeatability without wasting time. By utilizing customizable and flexible dosing options offered by many automatic titrators, the titrator looks at the rate of mV change throughout the titration to determine the dosing speed and size.
By doing this, larger volumes will be dosed more frequently at the beginning of the titration since the potential change is small. As the reaction approaches the endpoint, the mV potential starts to change more dramatically per dose. As a result, the titrator proportionally scales down the dose size and increases the time between doses.
Dosing a larger volume of titrant in the beginning of the titration, and less at the end, keeps the speed of each titration to a minimum while ensuring high resolution around the endpoint. Automating the dosing and endpoint detection allows analysts to perform other lab duties.
The Proof is in Potentiometry
 Hanna Instruments recently worked with a manufacturer at a metal plating facility to automate their bath titration. The quality control analysts were performing manual titrations for the measurement of acidity in the chrome baths, as well as the acidity and nickel in the nickel baths.
Hanna Instruments recently worked with a manufacturer at a metal plating facility to automate their bath titration. The quality control analysts were performing manual titrations for the measurement of acidity in the chrome baths, as well as the acidity and nickel in the nickel baths.
Many of the baths had colored sample matrices such as a dark, murky hue, so the customer was forced to use a very small sample size to try and discern their endpoints using color indicators. This is a large facility, so multiple technicians work in the lab.
Since they used a color indicator, their titration results were open to interpretation between many different technicians. The lab manager noticed that they were obtaining inconsistent results across shifts, and this resulted in inconsistencies in their finished products.
Hanna Instruments worked with the manufacturer to automate their manual titrations using our Automatic Potentiometric Titrator HI932. Once the titrations were automated, results became consistent across shifts and the analysts were able to perform other tasks while a titration was running.
This allowed them to run tests more frequently and maintain higher quality baths, while also reducing the amount of rejected finished products. Before, the lab would become backlogged with samples, causing the employees to have to stay late or rush through them. Now, the samples are titrated along with other analyses in a fraction of the time.
Hanna Instruments Titration Package
The Automatic Titrator Nickel Package HI932 offers everything you need to accomplish a smooth transition from manual titrations to automated titrations. Our HI932 package includes:
- The HI932 automatic potentiometric titrator with two stirring assemblies and two 40,000-step piston-driven dosing pumps and burettes
- A pH electrode, an ORP electrode, and cupric ion selective electrode
- Titrants and reagents for acidity, hypophosphite, and nickel titrations
- Calibration buffers and maintenance solutions for pH electrodes
- On-site installation and training, including on-site method optimization
That's why we've dedicated our blog as a helpful resource for you to use! Catch up on the latest products, explore industry trends, discover testing tips, learn how to improve results, and more. Got questions? Email sales@hannainst.com.

