The harvest season is here. We realize that this is the busiest and most critical period of the year for winemakers. Organizing the harvest and planning the work is vital to a successful and trouble-free vintage. Preparation for the analytical measurements relied upon to make winemaking decisions can sometimes be missed. To ensure success, it is important to prepare the equipment necessary to test pH, SO2, TA and Brix.
Here’s our Winemakers' Guide to making sure that you are ready for the season.
- Check Your Buffers, Solutions, and Reagents
- Prepare and Check Your Electrodes
- Prepare Automatic Titrators and Related Equipment
- Refractometers
1. Check Your Buffers, Solutions, and Reagents
All buffers, solutions and reagents that open for more than six months should be replaced. Make sure that you have fresh product on hand. Calibrations and titrations performed are only as good as the buffers, solutions, and reagents being used.
Calibration buffers: Buffers, by definition, should resist change. But overtime, an opened bottle will degrade and the value on the bottle is not the actual value of the solution.
Hanna Tip: When measuring pH in wine, it is best to calibrate to two points. Most meters will allow for pH 7.01 and pH 4.01 calibration. Ideally the calibration should be done to pH 7.01 and pH 3.00. This allows for calibration to bracket the expected value which for wine is less than pH 4. Some meters are pre-programmed or allow for a custom calibration point (i.e. pH 3.00).
Electrode Cleaning Solution
A clean electrode is critical to both an accurate and stable pH reading. Any stains or coatings on the sensitive glass surface cause a shift in potential generated by the pH electrode in a solution. Clogging of the junction (barrier between the inside of the electrode and the sample) increases resistance and affects the junction potential. Any coatings on the glass or clogging of the junction will result in sluggish and erratic readings. Electrodes should be cleaned periodically.
Hanna Tip: Specially formulated cleaning solutions are available to remove stains and deposits from the electrode. These solutions are preferred over a general purpose cleaning solution since they are formulated for a specific purpose.
Electrode Storage Solution
A storage solution is designed to keep the pH bulb hydrated and to maintain free flowing junction. The hydration of a pH electrode takes 3-4 hours to completely form. Without this layer, the pH calibration will drift over time. It is also important to maintain a free flowing junction. If the junction is allowed to dry out then the diffusion of the internal electrolyte through the junction will be impeded. Not only will the junction potential be affected but also the stability/response of the electrode. Properly stored electrodes exhibit higher accuracy and have a longer lifespan.
Hanna Tip: Never store a pH or ORP electrode in purified (deionized, distilled, reverse osmosis) water. Purified water increases the diffusion of reference electrolyte into the solution and causes water to move into the reference cell by osmosis. Both will change the composition of reference electrolyte. If the solution cannot be replaced then the probe will.
Hanna Tip: Storage solutions are formulated to minimize any concentration gradient between the internal reference and the sample but also prevent any organic growth.
Electrode Fill Solution
For a refillable pH or ORP electrode, a refill solution or electrolyte, is available. It is important that adequate level be maintained in order to provided adequate amount of head pressure. A positive head pressure (refill cap removed) allows for the flow of electrolyte through the junction into the sample. This is important since the ions in the electrolyte electrically connect the meter and electrode with the wine sample being tested. Levels of electrolyte should regularly be checked.

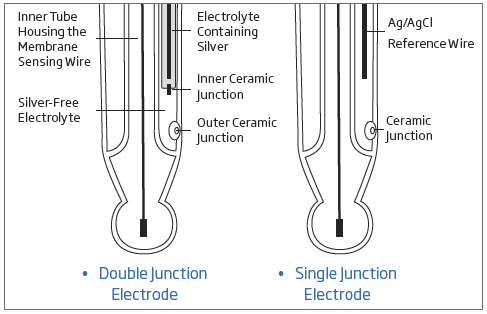
Hanna Tip: Single junction pH and ORP electrodes will use a potassium chloride (KCl) solution saturated with silver chloride (AgCl) while double junction electrodes use only KCl. The diagram below that will help identify what type of electrode that you have.
Hanna Buffers and Solutions for Wine:
- HI7007L pH 7.01 buffer (500 ml)
- HI7004L pH 4.01 buffer (500 ml)
- HI5003 pH 3.00 buffer (500 ml)
- HI70300L Electrode storage solution (500 mL)
- HI70635L Cleaning solution for wine deposits (500 mL)
- HI70636L Cleaning solution for wine stains (500 mL)
- HI7082 Electrode filling solution for double junction electrodes (4 X 30 mL)
- HI7071 Electrode filling solution for single junction electrodes (4 X 30 mL)

2. Prepare and Check Your Electrodes
Having fresh solutions is essential but it is equally important that the electrodes be checked for functionality.
pH / TA / Formal Number (Nitrogen) determinations all use a pH electrode. The following is a quick way to determine the overall condition of a pH electrode. The overall condition is based on offset (pH7.01 mV value) and slope (difference between pH 7.01 mV and pH 4.01 mV). Both are of equal importance and any deviation from recommended values will result in erroneous readings of the sample.
- The offset and slope of a pH electrode should be checked with fresh buffers. If your meter has a Good Laboratory Practice (GLP) option then this will provide the data after calibration.
- If your meter does not have a GLP option then any meter with a mV mode can be used to check the pH electrode.
- When a pH electrode is placed in pH 7.01 buffer the theoretical mV observed should be 0 mV. Because of variations in the glass, aging, and conditioning the offset voltage will differ from 0 mV. An acceptable offset voltage is +/- 30 mV. Most meters will calibrate to values as high as +/- 60 mV for pH 7.01 but the accuracy of the measurement will diminish. Any values outside +/- 30 mV range indicates that the probe needs to be cleaned and hydrated. If not corrected then for a refillable probe the reference fill solution should be changed. If still not corrected then a new pH electrode should be considered.
- The ideal slope for a pH electrode is 100% or 59.16 mV change per pH unit @ 25 ºC. For pH 4 that is three pH units away from pH 7 this represent 177.48 mV difference (59.16 x 3). The absolute minimum slope is 85% or 150 mV difference (177.48 x 0.85). Again, this is the absolute minimum slope with many government regulated industries with a higher slope requirement. A 90% slope is equal to a 160 mV difference for solutions being measured at 25 ºC. These values change with temperature as calculated by the Nernst equation.
Video: Slope and Offset (3 minutes)
Additional pH Electrode Tips
- pH electrodes should be calibrated before use. Periodic calibration, if not daily, should be performed to obtain a high accuracy of measurement.
- If readings drift excessively and are slow to stabilize (more than 30 seconds) the electrode may need to be cleaned or the electrode fill solution may need to be changed
- Ensure that fill solution is more than ¾ full
SO2 potentiometric titrations use an ORP electrode for determining the equivalence endpoint. Even though the endpoint does not rely on a specific value to be reached (i.e. TA titration to pH 8.2) the mV response of an ORP electrode will provide an indication to its sensitivity in measuring a redox potential. It is important to periodically check the ORP electrode using an ORP test solution.
To check an ORP electrode the probe is placed in an ORP test solution of a known value. The HI7021L is a 500 ml bottle of 240 mV @ 25 ºC ORP test solution. A properly functioning electrode will read 240 mV +/- 20 mV. Readings outside this range indicate that the platinum tip/band needs to be polished or the electrolyte fill solution needs to be changed. If the ORP electrode cannot be brought within range then it should be replaced.
Hanna Tip: Very fine sand paper (i.e. 2000 grit) can be used to polish a tarnished ORP tip or band. The ORP sensing portion of the electrode should be shiny and not tarnished.
Need a new electrode?
Hanna Instruments offers a variety of pH and ORP electrodes for various applications. What makes an electrode specific for any particular application is the design criteria required including body type, junction material, type of junction, fill solution, and for pH electrodes the type of sensitive glass used. For analytical measurements of wine juice and must it is important to have an electrode that resists clogging of the junction by the solids in the sample. The following are the pH and ORP electrodes recommended for pH/mV meters and titration systems:
pH
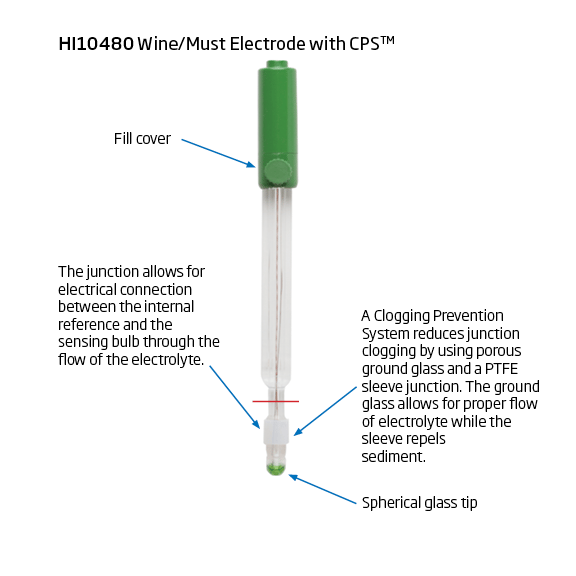
The HI1048 series of electrodes are for wine juice and must. It is a glass body, double junction, refillable pH electrode. At the heart of the HI1048 is the unique outer junction made of PTFE.
CPS Sleeve Junction
CPS™ (Clogging Prevention System) is an innovation in electrode technology. Conventional pH electrodes use ceramic junctions that clog quickly when used in wine. When the junction is clogged, the electrode does not function. CPS™ technology utilizes the porousness of ground glass coupled with a PTFE sleeve to prevent clogging of the junction. The ground glass allows proper flow of the liquid, while the PTFE sleeve repels dirt. As a result, pH electrodes with CPS™ stay fresh up to 20 times longer than conventional electrodes.
Double Junction Reference
A double junction electrode has an internal compartment surrounding the reference wire. Silver ions are present in the electrolyte of the internal compartment, which houses the Ag/AgCl reference wire; the electrolyte outside this compartment is silver free. The double junction design means that virtually no silver from the electrode enters the sample. This design allows measurement in applications where silver ions in the sample are undesirable or silver precipitates on the junction are likely to form.
 Refillable
Refillable
The HI1048 is a refillable probe. Since it is a double junction pH electrode the fill solution is the HI7082 3.5M KCl. This solution does not contain any silver as with single junction electrode. The absence of silver will prevent any silver precipitate from forming at the junction surface and clogging it. Clogging of the junction will result in drifty and erratic readings.
HI1048 Probe by Connector Type (meter dependent)
The HI1048 electrodes are available with a variety of connectors based on the meter being used.
 HI1048B (BNC Connector)
HI1048B (BNC Connector)
This connector is universal and can be used with any meter that has a BNC connector. It is the original probe supplied with the HI84102 and HI 84502 acidity mini titrator. It was also supplied with the HI9126V portable pH/mV meter.
 HI1048P (BNC and Pin Connectors)
HI1048P (BNC and Pin Connectors)
This probe is the same as the HI1048B but has a pin connector used to enable the calibration check feature of the HI222W and HI2222W benchtop pH/mV meters.
HI1048D (DIN Connector)
This connector is proprietary for the HI99111 portable pH/Temperature meter. The HI1048D has a built in temperature sensor for temperature compensated measurements.
HI10480 (3.5 mm Pin Connector)
 The edge digital pH electrode contains a built-in microchip that stores sensor type, serial number and calibration information including date, time, offset, slope, probe condition and buffers used. The HI10480 has a built in temperature sensor for temperature compensated measurements. This connector is proprietary and is used with the HI2020, HI2030, and HI2040 multiparameter edge meters. The HI10480 can also be used with the HI2002 edge dedicated pH meter. The HI10480 is the supplied pH electrode with the HI2020W winemaker’s kit.
The edge digital pH electrode contains a built-in microchip that stores sensor type, serial number and calibration information including date, time, offset, slope, probe condition and buffers used. The HI10480 has a built in temperature sensor for temperature compensated measurements. This connector is proprietary and is used with the HI2020, HI2030, and HI2040 multiparameter edge meters. The HI10480 can also be used with the HI2002 edge dedicated pH meter. The HI10480 is the supplied pH electrode with the HI2020W winemaker’s kit.
ORP Electrodes
Much like a pH electrode, ORP electrodes also have their own design criteria. Since the ORP electrode is used with the same type of samples in wine testing as a pH electrode, the favorable criteria are similar.
The HI3148 is a double junction, refillable ORP electrode with a platinum ring for a sensor. The HI1048 also has the CPS™ (Clogging Prevention System) technology with the same unique outer junction made of PTFE as the HI1048 pH electrode.
The HI3148B has a BNC connector and can be used with any titrator that has a BNC input. The HI3148B is supplied with the HI84500 free and total sulfur dioxide titrator. It was also supplied with the HI84100 mini titrator.
3. Prepare Automatic Titrators and Related Equipment
Click the part number below for a complete list of titrants, reagents and replacement parts for Hanna Instruments wine specific titration system.
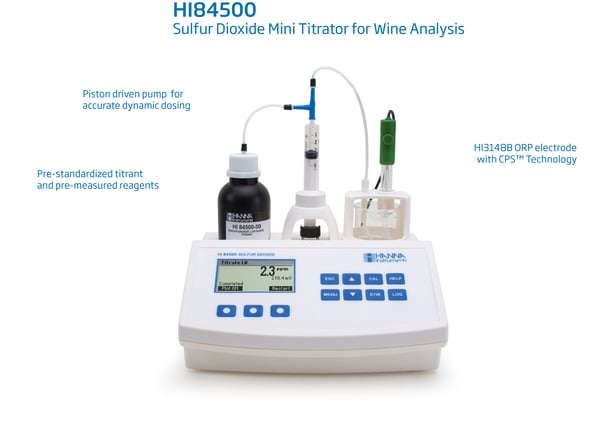
HI84500 – Piston driven syringe Free and Total SO2 Mini Titrator
Download quick guide for HI84500
HI84502 – Piston driven syringe TA Mini Titrator
Download quick guide for HI84502
Follow manufacturer recommendations for calibration and maintenance.
Note to Hanna Customers
- HI84100 and HI84102 users should replace old tubing and calibrate the pump before the season start.
- Visit our website or contact us for a complete range of solutions, buffers and reagents that are available.
4. Refractometers
A well designed refractometer needs very little maintenance. If you are using a mechanical refractometer then you may want to consider upgrading to a digital refractometer for improved accuracy and ease-of-use.
The HI96811 and HI96813 wine specific digital refractometers are manufactured by Hanna.
Video: Wine Refractometers (3 minutes)
HI96811 measures from 0 to 50% Brix with an accuracy of +/- 0.2% Brix.
HI96813 is the same as the HI96811 but also has an algorithm to predict the potential alcohol from the refractive index of the sample. The potential alcohol range is 0 to 25% V/V.
Both meters convert the refractive index of a wine, juice or must sample to % Brix. This conversion is based on the tables found in the ICUMSA Methods Book (International Commission for Uniform Methods of Sugar Analysis) that documents the changes in refractive index with temperature for a percent by weight sucrose solution. Since the majority of sugar in grape juice and must is fructose and glucose instead of sucrose, the reading is sometimes referred to as “Apparent Brix”. Common features for both meters are::
- Reports sugar content as % Brix.
- Simple operation with only two buttons: one button is to calibrate with distilled or deionized water and the other to take a measurement
- Sample size as small as two metric drops or about 100 μL.
- All readings are automatically compensated for temperature variations with a 1.5 second response time
- The sealed flint glass prism and stainless steel well are easy to clean - just wipe with a soft cloth in preparation for the next sample
All the best for a successful harvest!
That's why we've dedicated our blog as a helpful resource for you to use! Catch up on the latest products, explore industry trends, discover testing tips, learn how to improve results, and more. Got questions? Email sales@hannainst.com.